Isotopes
- These are elements which have the same atomic number but different atomic mass.
- They have the same atomic number because the number of protons that are inside their nuclei remains the same.
- But, they have different atomic mass because the number of neutrons that are also inside their nuclei is different.
- As the number of protons inside nuclei remains same, therefore the overall charge of the elements also remains same as in isotopes: no of protons = no of electrons.
- Hence, as isotopes overall charge remains neutral, therefore their chemical properties will also remain identical.
Therefore, Isotopes are chemically same but physically different.
Examples of isotopes are:
1) Isotopes of hydrogen
2) Isotopes of carbon
Isobars
- These are elements which have same atomic mass but a different atomic number.
- Hence, as isobars have different atomic number, therefore, they have a different number of electrons, and their chemical properties are different.
- The light nuclei have unstable isobars.
- Heavy nuclei have stable isobars and these occur in pairs.
- If the number of protons of one isobar matches with that of another, then they are called as mirror-nuclides of each other
Therefore, Isobars are chemically different but physically same.
examples:
Isotones
These are elements having the same number of neutrons.
You Might Also Like
Enjoy your high school with - High School Pedia : www.highschoolpedia.com



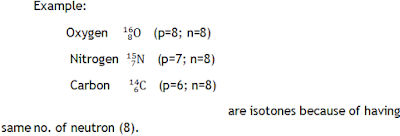
Good.
ReplyDeleteYou have shared a nice article here about the Isotopes, Isobars and Isotones. Your article is very informative and useful to know more about the Isotopes, Isobars and Isotones. If anyone looking for the Physics Tutor Online, teacherlookup is the best choice.
ReplyDelete
ReplyDeleteKara's Orchard CBD Gummies Tyrosine is an amino destructive that prompts the headway of levodopa, which is changed over into dopamine.
https://sites.google.com/view/karasorchardcbdgummiesuk-facts/